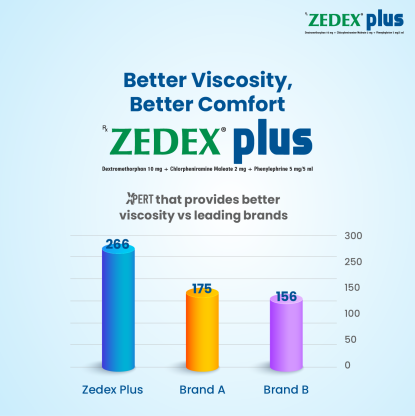
Conquer Cold and Cough with Dextromethorphan Hydrobromide, Chlorpheniramine Maleate and Phenylephrine Hydrochloride!
1. Oh S, Agrawal S, Sabir S, Taylor A. Dextromethorphan.
2. Eccles, R., Fietze, I. and Rose, U.-B. (2014) Rationale for Treatment of Common Cold and Flu with Multi-Ingredient Combination Products for Multi-Symptom Relief in Adults. Open Journal of Respiratory Diseases, 4, 73-82. http://dx.doi.org/10.4236/ojrd.2014.43011.
3. Zedex plus PI 2024
 Where to use?
Where to use?
- Dextromethorphan, Chlorpheniramine, and Phenylephrine are commonly used in combination to treat symptoms associated with the common cold and upper respiratory tract infections.
- They are typically used to relieve:
Cough: Dextromethorphan acts as a cough suppressant.
Nasal Congestion: Phenylephrine helps to reduce nasal congestion by constricting blood vessels.
Runny Nose and Sneezing:
Chlorpheniramine alleviates symptoms such as runny nose and sneezing by blocking histamine receptors.
1. Oh S, Agrawal S, Sabir S, Taylor A. Dextromethorphan.
2. Livier Castillo J, et al. The Use and Efficacy of Oral Phenylephrine Versus Placebo Treating Nasal Congestion Over the Years on Adults: A Systematic Review. Cureus. 2023 Nov 19;15(11):e49074.
3. Zedex plus PI 2024
 How to use?
How to use?
- Shake the syrup bottle well before use to ensure that the ingredients are evenly distributed
- Measure the dose with a measuring cap and ensure correct oral doses.
- Check the instructions on the label thoroughly before use.
- For Adults and children >12 years -10ml 2-3 times a day.
- ZEDEX PLUS can be taken with or without food, but taken as per physicians direction.
1. Zedex plus PI 2024
 Possible Side effects
Possible Side effects
- Dizziness
- Dry mouth
- Headache
- Sleep disturbances
- Palpitations
- Rashes or itching
- Blurred Vision
- GI disturbance
(Nausea-vomiting, constipation, heartburn or anorexia)
1. Zedex plus PI 2024
 Safety Advises
Safety Advises
Alcohol:
Caution is advised when consuming alcohol with ZEDEX PLUSRx. Please consult your doctor.
Pregnancy and Lactation:
Please consult your doctor if you are pregnant, breastfeeding, or planning to conceive during the treatment. This medication may cause fetal harm or be secreted in breast milk. Your doctor will weigh the benefits and potential risks before prescribing it to you.
Caution:
Caution should be maintained in patients with history of cardiac disorders, diabetes or peripheral vascular disease.
Driving:
Please drive with caution while on the medication and drive only if necessary. ZEDEX PLUSRx may cause your vision to blur and may cause dizziness, which involves risk in driving.
1. Zedex plus PI 2024
 Why this combination?
Why this combination?
CHLORPHENIRAMINE; DEXTROMETHORPHAN; PHENYLEPHRINE is a combination of an antihistamine, a cough suppressant, and a decongestant. When combined, these medications provide a multi-symptom approach to treating cold and allergy symptoms.1
Here’s how they complement each other:2,3,4
Cough Suppression:
Dextromethorphan helps manage coughs, which can be particularly bothersome during colds.
Allergy Relief:
Chlorpheniramine addresses the histamine-related symptoms, reducing sneezing, itching, and runny nose.
Nasal Congestion Relief:
Phenylephrine helps clear nasal congestion, making it easier to breathe.
1. Chlorpheniramine; Dextromethorphan; Phenylephrine oral solution or syrup. Available at: https://my.clevelandclinic.org/health/drugs/ 19488-chlorpheniramine-dextromethorphan-phenylephrine-oral-solution-or-syrup. Accessed on 15th July 2024.
2. Oh S, Agrawal S, Sabir S, Taylor A. Dextromethorphan.
3. Livier Castillo J, et al. The Use and Efficacy of Oral Phenylephrine Versus Placebo Treating Nasal Congestion Over the Years on Adults: A Systematic Review. Cureus. 2023 Nov 19;15(11):e49074.
4. Zedex plus PI 2024.
Editor's Pick
ICS Guidelines for cough type
ICS Guidelines for cough type
ICS Guidelines for cough typeDid You Hear? DXM 15 Can Transform Your Patients' Cough
Did You Hear? DXM 15 Can Transform Your Patients' Cough
Did You Hear? DXM 15 Can Transform Your Patients' CoughThe Cough Combo That Everyone Approves
The Cough Combo That Everyone Approves
The Cough Combo That Everyone ApprovesDextromethorphan Fixed Dose Combinations now recommended by Indian Chest Society
Dextromethorphan Fixed Dose Combinations now recommended by Indian Chest Society
ZEDEX PLUS with Dextromethorphan Fixed-Dose Combinations offers proven efficacy and superior patient outcomes. Even the Indian Chest Society endorses it!Dextromethorphan Fixed Dose Combinations now recommended by Indian Chest Society
ZEDEX PLUS’s syrup ranks at the top in all organoleptic properties
ZEDEX PLUS’s syrup ranks at the top in all organoleptic properties
ZEDEX PLUS soars high in scoring top ranks amongst other players in the market. 240 patients also say so!ZEDEX PLUS’s syrup ranks at the top in all organoleptic properties
Taste the Difference with Mouthfeel that Delivers!
Taste the Difference with Mouthfeel that Delivers!
Don’t settle let your patients settle for less—choose ZEDEX PLUS for a cough syrup that's smooth, effective, and loved by 240 patients!Taste the Difference with Mouthfeel that Delivers!
ZEDEX PLUS is winning over patients with its taste and colour
ZEDEX PLUS is winning over patients with its taste and colour
Wave goodbye to that annoying cough with ZEDEX PLUS! Not only does it work wonders, but it also tastes amazing.ZEDEX PLUS is winning over patients with its taste and colour
X-ray patterns and insights for different cough categories
X-ray patterns and insights for different cough categories
Analyzing X-rays across various categories of cough patients, such as smokers' cough, bronchospastic cough (Asthma), infectious cough (Pneumonia), and postnasal drip cough—offers valuable insights into each condition.X-ray patterns and insights for different cough categories
The impact of long-lasting coughs on diverse patient population
The impact of long-lasting coughs on diverse patient population
Chronic coughs worsen health for those with diabetes, GERD, asthma, COPD, heart issues, allergies, & the elderly. They disrupt blood sugar, increase reflux, trigger asthma attacks, obstruct breathing, stress the heart, & heighten allergy sensitivity.The impact of long-lasting coughs on diverse patient population
Impact of untreated long-lasting cough in patients
Impact of untreated long-lasting cough in patients
Chronic cough affects more women than men and can lead to fatigue, sleep disturbances, musculoskeletal pain & psychological issues like anxiety. It may worsen asthma or GERD. Severe coughing episodes can cause rib fractures and urinary incontinence.Impact of untreated long-lasting cough in patients
Inspiratory Muscle Training Improves Cough Strength in Frail Older Adults
Inspiratory Muscle Training Improves Cough Strength in Frail Older Adults
A recent randomized controlled trial has shown that inspiratory muscle training (IMT) significantly enhances cough strength in older adults with frailty, suggesting its potential as a targeted intervention for this vulnerable population.
The study involved 60 frail older adults, with 52 completing the trial. Participants were randomly assigned to either an IMT group, which performed inspiratory muscle exercises alongside general exercise training, or a control group that engaged in general exercise training alone.
IMT participants used a threshold device set at 30% of their maximum inspiratory pressure, performing 30 breaths twice daily over eight weeks.
The primary outcome, cough peak flow (CPF), demonstrated notable improvement in the IMT group, with an average increase of 28.7 ± 44.4 L/min compared to a decrease of -7.4 ± 26.6 L/min in the control group. The mean difference between groups was 36.3 L/min (95% CI: 16.7–55.9), with a large effect size of 0.99.
These findings indicate that IMT is a practical and effective approach to strengthening respiratory muscles, leading to improved cough function in frail older adults. Enhanced cough strength can play a critical role in reducing the risk of complications such as aspiration and respiratory infections, offering a valuable addition to care strategies for this demographic.
Inspiratory Muscle Training Improves Cough Strength in Frail Older Adults
Inspiratory Muscle Training Improves Cough Strength in Frail Older Adults
A recent randomized controlled trial has shown that inspiratory muscle training (IMT) significantly enhances cough strength in older adults with frailty, suggesting its potential as a targeted intervention for this vulnerable population.
The study involved 60 frail older adults, with 52 completing the trial. Participants were randomly assigned to either an IMT group, which performed inspiratory muscle exercises alongside general exercise training, or a control group that engaged in general exercise training alone.
IMT participants used a threshold device set at 30% of their maximum inspiratory pressure, performing 30 breaths twice daily over eight weeks.
The primary outcome, cough peak flow (CPF), demonstrated notable improvement in the IMT group, with an average increase of 28.7 ± 44.4 L/min compared to a decrease of -7.4 ± 26.6 L/min in the control group. The mean difference between groups was 36.3 L/min (95% CI: 16.7–55.9), with a large effect size of 0.99.
These findings indicate that IMT is a practical and effective approach to strengthening respiratory muscles, leading to improved cough function in frail older adults. Enhanced cough strength can play a critical role in reducing the risk of complications such as aspiration and respiratory infections, offering a valuable addition to care strategies for this demographic.
Gefapixant Reduces Cough-Induced Stress Urinary Incontinence in Women with Chronic Cough
Gefapixant Reduces Cough-Induced Stress Urinary Incontinence in Women with Chronic Cough
A phase 3b randomized controlled trial has demonstrated that gefapixant significantly reduces episodes of cough-induced stress urinary incontinence (CSUI) in women with refractory or unexplained chronic cough, marking a breakthrough in treating this challenging condition.
The study included 375 women aged 18 and older with chronic cough lasting at least one year and CSUI persisting for over three months.
Participants were randomized to receive gefapixant 45 mg twice daily or placebo for 12 weeks. The primary outcome was the percentage change in daily CSUI episodes over seven days at week 12.
Results showed a -52.8% reduction in CSUI episodes with gefapixant compared to -41.1% with placebo, yielding an estimated treatment difference of -11.7% (P = 0.004).
Gefapixant also demonstrated consistent safety, with the most common adverse events being taste-related disturbances. Adverse events occurred in 70% of the gefapixant group compared to 37% in the placebo group, aligning with prior studies of the drug.
This trial establishes gefapixant as the first treatment to demonstrate efficacy in reducing CSUI episodes among women with chronic cough, addressing a significant unmet medical need. The findings highlight gefapixant’s potential as a dual-action therapy, improving both chronic cough and its impact on urinary health, with a manageable safety profile.
Gefapixant Reduces Cough-Induced Stress Urinary Incontinence in Women with Chronic Cough
Gefapixant Reduces Cough-Induced Stress Urinary Incontinence in Women with Chronic Cough
A phase 3b randomized controlled trial has demonstrated that gefapixant significantly reduces episodes of cough-induced stress urinary incontinence (CSUI) in women with refractory or unexplained chronic cough, marking a breakthrough in treating this challenging condition.
The study included 375 women aged 18 and older with chronic cough lasting at least one year and CSUI persisting for over three months.
Participants were randomized to receive gefapixant 45 mg twice daily or placebo for 12 weeks. The primary outcome was the percentage change in daily CSUI episodes over seven days at week 12.
Results showed a -52.8% reduction in CSUI episodes with gefapixant compared to -41.1% with placebo, yielding an estimated treatment difference of -11.7% (P = 0.004).
Gefapixant also demonstrated consistent safety, with the most common adverse events being taste-related disturbances. Adverse events occurred in 70% of the gefapixant group compared to 37% in the placebo group, aligning with prior studies of the drug.
This trial establishes gefapixant as the first treatment to demonstrate efficacy in reducing CSUI episodes among women with chronic cough, addressing a significant unmet medical need. The findings highlight gefapixant’s potential as a dual-action therapy, improving both chronic cough and its impact on urinary health, with a manageable safety profile.
Indoor Radon Decay Products Linked to Increased Cough and Phlegm in COPD Patients
Indoor Radon Decay Products Linked to Increased Cough and Phlegm in COPD Patients
A recent study highlights the association between exposure to radon decay products, measured as particle radioactivity (PR), and worsening respiratory symptoms in patients with chronic obstructive pulmonary disease (COPD).
The study involved 141 male former smokers with COPD who participated in up to four one-week seasonal assessments. Researchers measured indoor and ambient particulate matter (PM2.5) and black carbon (BC), alongside PR levels, using α-radiation activity on PM2.5 filters.
Respiratory symptoms, including cough, phlegm, and shortness of breath, were evaluated through the St. George’s Respiratory Questionnaire (SGRQ), which also assessed health-related quality of life (HRQL).
Results revealed that indoor radon decay products were significantly associated with increased cough (31.1% per interquartile range [IQR], 95% CI: 8.8%–57.8%) and suggestively associated with phlegm (13.0% per IQR, 95% CI: -2.5%–31.0%).
A modest increase in SGRQ symptom scores (1.2 units per IQR, 95% CI: -0.3–2.6) was also observed, though it did not reach statistical significance. These effects persisted after adjusting for indoor PM2.5 and BC.
The findings underscore the impact of indoor radon decay exposure on respiratory health in COPD patients, emphasizing the need for improved indoor air quality to mitigate symptom severity and enhance quality of life in this vulnerable population.
Indoor Radon Decay Products Linked to Increased Cough and Phlegm in COPD Patients
Indoor Radon Decay Products Linked to Increased Cough and Phlegm in COPD Patients
A recent study highlights the association between exposure to radon decay products, measured as particle radioactivity (PR), and worsening respiratory symptoms in patients with chronic obstructive pulmonary disease (COPD).
The study involved 141 male former smokers with COPD who participated in up to four one-week seasonal assessments. Researchers measured indoor and ambient particulate matter (PM2.5) and black carbon (BC), alongside PR levels, using α-radiation activity on PM2.5 filters.
Respiratory symptoms, including cough, phlegm, and shortness of breath, were evaluated through the St. George’s Respiratory Questionnaire (SGRQ), which also assessed health-related quality of life (HRQL).
Results revealed that indoor radon decay products were significantly associated with increased cough (31.1% per interquartile range [IQR], 95% CI: 8.8%–57.8%) and suggestively associated with phlegm (13.0% per IQR, 95% CI: -2.5%–31.0%).
A modest increase in SGRQ symptom scores (1.2 units per IQR, 95% CI: -0.3–2.6) was also observed, though it did not reach statistical significance. These effects persisted after adjusting for indoor PM2.5 and BC.
The findings underscore the impact of indoor radon decay exposure on respiratory health in COPD patients, emphasizing the need for improved indoor air quality to mitigate symptom severity and enhance quality of life in this vulnerable population.
Diaphragmatic Breathing Significantly Improves Chronic Cough in GERD Patients
Diaphragmatic Breathing Significantly Improves Chronic Cough in GERD Patients
A randomized controlled trial has demonstrated that deep diaphragmatic breathing (DEP) training significantly enhances treatment outcomes for patients suffering from gastroesophageal reflux-induced chronic cough (GERC).
Sixty patients were divided into two groups: one receiving standard medication for GERC and the other undergoing additional DEP training.
After eight weeks, both groups experienced symptom improvement, but the intervention group, which practiced DEP, showed a markedly higher cough resolution rate (94%) compared to the control group (77%, p = 0.041).
DEP training also resulted in significantly better scores in the gastroesophageal reflux diagnostic questionnaire (GerdQ), generalized anxiety disorder scale (GAD-7), Pittsburgh sleep quality index (PSQI), and Leicester cough questionnaire (LCQ). Importantly, patients who practiced DEP displayed improved diaphragmatic muscle activity, measured by surface electromyography (sEMG), during both deep and quiet breathing.
This research highlights the therapeutic potential of diaphragmatic breathing as a valuable adjunct to standard medical treatment for GERC patients. Improvements in cough symptoms, anxiety levels, sleep quality, and overall quality of life suggest that DEP training may offer a non-invasive, low-cost approach to managing chronic cough caused by gastroesophageal reflux disease.
These findings provide a strong rationale for incorporating breathing exercises into the management plans for patients with GERC, enhancing symptom control beyond conventional treatments alone.
Diaphragmatic Breathing Significantly Improves Chronic Cough in GERD Patients
Diaphragmatic Breathing Significantly Improves Chronic Cough in GERD Patients
A randomized controlled trial has demonstrated that deep diaphragmatic breathing (DEP) training significantly enhances treatment outcomes for patients suffering from gastroesophageal reflux-induced chronic cough (GERC).
Sixty patients were divided into two groups: one receiving standard medication for GERC and the other undergoing additional DEP training.
After eight weeks, both groups experienced symptom improvement, but the intervention group, which practiced DEP, showed a markedly higher cough resolution rate (94%) compared to the control group (77%, p = 0.041).
DEP training also resulted in significantly better scores in the gastroesophageal reflux diagnostic questionnaire (GerdQ), generalized anxiety disorder scale (GAD-7), Pittsburgh sleep quality index (PSQI), and Leicester cough questionnaire (LCQ). Importantly, patients who practiced DEP displayed improved diaphragmatic muscle activity, measured by surface electromyography (sEMG), during both deep and quiet breathing.
This research highlights the therapeutic potential of diaphragmatic breathing as a valuable adjunct to standard medical treatment for GERC patients. Improvements in cough symptoms, anxiety levels, sleep quality, and overall quality of life suggest that DEP training may offer a non-invasive, low-cost approach to managing chronic cough caused by gastroesophageal reflux disease.
These findings provide a strong rationale for incorporating breathing exercises into the management plans for patients with GERC, enhancing symptom control beyond conventional treatments alone.
Chronic Cough Triggers Vary by Etiology and Are Only Partially Linked to Capsaicin Sensitivity
Chronic Cough Triggers Vary by Etiology and Are Only Partially Linked to Capsaicin Sensitivity
A comprehensive study has revealed the diverse profile of cough triggers in chronic cough patients and their limited correlation with capsaicin cough sensitivity.
In a cross-sectional analysis of 1,211 patients with chronic cough, 91.4% reported experiencing at least one trigger. Chemical triggers were most prevalent (66.9%), followed by thermal exposure (50.6%), mechanical triggers (48.2%), and meal-related triggers (21.2%).
While chemical triggers were consistent across different cough etiologies, meal and mechanical triggers were more common in refractory chronic cough, with meal triggers particularly associated with gastroesophageal reflux-related cough.
Refractory chronic cough patients reported the highest prevalence of triggers (97.1%). Capsaicin challenge tests conducted on 254 participants showed a mild correlation between the number of triggers, particularly chemical ones, and capsaicin cough sensitivity. However, these findings suggest that cough hypersensitivity in chronic cough encompasses a broader spectrum of mechanisms beyond capsaicin sensitivity.
The study highlights the need for personalized approaches in managing chronic cough, considering the varied triggers and their etiology-specific profiles.
Chronic Cough Triggers Vary by Etiology and Are Only Partially Linked to Capsaicin Sensitivity
Chronic Cough Triggers Vary by Etiology and Are Only Partially Linked to Capsaicin Sensitivity
A comprehensive study has revealed the diverse profile of cough triggers in chronic cough patients and their limited correlation with capsaicin cough sensitivity.
In a cross-sectional analysis of 1,211 patients with chronic cough, 91.4% reported experiencing at least one trigger. Chemical triggers were most prevalent (66.9%), followed by thermal exposure (50.6%), mechanical triggers (48.2%), and meal-related triggers (21.2%).
While chemical triggers were consistent across different cough etiologies, meal and mechanical triggers were more common in refractory chronic cough, with meal triggers particularly associated with gastroesophageal reflux-related cough.
Refractory chronic cough patients reported the highest prevalence of triggers (97.1%). Capsaicin challenge tests conducted on 254 participants showed a mild correlation between the number of triggers, particularly chemical ones, and capsaicin cough sensitivity. However, these findings suggest that cough hypersensitivity in chronic cough encompasses a broader spectrum of mechanisms beyond capsaicin sensitivity.
The study highlights the need for personalized approaches in managing chronic cough, considering the varied triggers and their etiology-specific profiles.
How would you rate this Medshorts
Thank you !
Your rating has been recorded.
-
-
Pediatric,Cough
-
-
Videos Speakers
Knowledge Hub
Q1: What are the active ingredients in ZEDEX PLUS?
A: ZEDEX PLUS contains a blend of active ingredients, including a cough suppressant, a decongestant, and an antihistamine.
Q2: How does ZEDEX PLUS work?
A: ZEDEX PLUS works by suppressing the cough reflex, reducing nasal congestion, and relieving symptoms of allergy such as runny nose and sneezing.
Q3: Is ZEDEX PLUS available over the counter?
A: Yes, ZEDEX PLUS is available over the counter, but it is recommended to consult with a healthcare provider before use.
Q4: What symptoms does ZEDEX PLUS treat?
A: ZEDEX PLUS treats cough, nasal congestion, runny nose, and other symptoms associated with the common cold and allergies.
Q5: How should I take ZEDEX PLUS?
A: Take ZEDEX PLUS as directed on the package or by your healthcare provider, usually with or without food.
Q6: What is the recommended dosage for adults?
A: The recommended dosage for adults is specified on the packaging or by your healthcare provider. Do not exceed the recommended dose.
Q7: Can I take ZEDEX PLUS with food?
A: Yes, ZEDEX PLUS can be taken with or without food.
Q8: What should I do if I miss a dose?
A: If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Do not double up to make up for the missed dose.
Q9: How long can I take ZEDEX PLUS?
A: Do not take ZEDEX PLUS for more than 7 days without consulting your healthcare provider.
Q10: Who should not take ZEDEX PLUS?
A: Individuals allergic to any ingredients, or those with certain medical conditions such as high blood pressure or heart disease, should avoid ZEDEX PLUS. Consult your healthcare provider for more information.
Q11: Can I take ZEDEX PLUS with other medications?
A: Always consult your healthcare provider before combining ZEDEX PLUS with other medications to avoid potential interactions.
Q12: Can I drink alcohol while taking ZEDEX PLUS?
A: Avoid alcohol while taking ZEDEX PLUS, as it can increase the risk of drowsiness and dizziness.
Q13: Is ZEDEX PLUS safe for children?
A: ZEDEX PLUS should be used in children only under the supervision of a healthcare provider and according to the recommended dosage.
Q14: How should I store ZEDEX PLUS?
A: Store ZEDEX PLUS at room temperature, away from light and moisture. Keep it out of reach of children.
Q15: What should I do if I accidentally overdose?
A: In case of an overdose, seek medical attention immediately. Overdose symptoms may include severe drowsiness, confusion, and difficulty breathing.
Q16: Can I share ZEDEX PLUS with someone else?
A: Do not share ZEDEX PLUS with others, as it is prescribed based on individual health conditions and needs.
Q17: How long does it take for ZEDEX PLUS to work?
A: ZEDEX PLUS typically starts to relieve symptoms within 30 minutes to an hour.
GGI-CO-A1-AQS-300033343-WM-G24-1630
-

Cough Algorithm: Simplify Cough Management in India
Read More
Want our representative to contact you ?
Below fields are needed for webinar purpose.