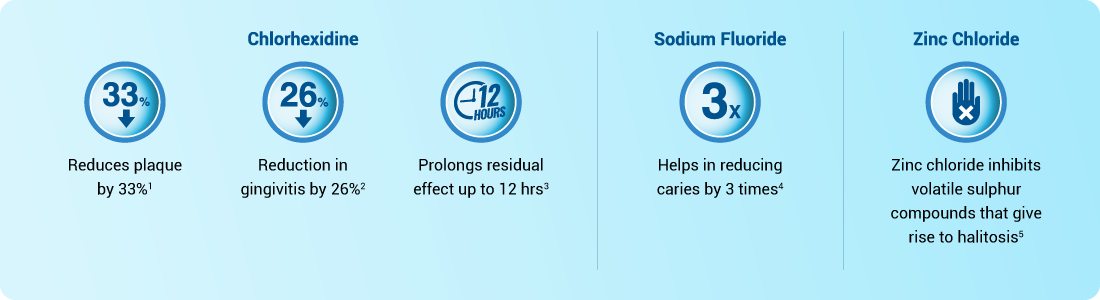
Triplex treatment with added advantage
Where to use Why Clohex ADS Mode of Action on stain Where to use?

Chlorhexidine in COVID-19

Expert's talk
Patient Education Videos
Abridged Prescribing Information
Clohex ADS (Antiseptic and Antiplaque Mouthwash)
Composition: Chlorhexidine Gluconate solution 0.2% w/v, Sodium Fluoride BP 0.05% w/v, Zinc chloride BP 0.09% w/v, Plasdone. Indications: Antiseptic and antiplaque mouth wash. For the prevention of dental plaque accumulation, dental caries and the prevention and treatment of gingivitis and periodontitis in reducing or preventing the formation of plaque, the causative factors of halitosis and the root caries. Dosage & administration: For adults and children above 10 years of age: To be used as required up to twice daily. Rinse the mouth thoroughly for about 1 minute with 10ml. The mouthwash should be expelled from the mouth after rinsing. Prior to dental surgery: Rinse the mouth thoroughly with 10ml for 1 minute. Treatment of gingivitis: A course of one month is recommended. Treatment of denture stomatitis: Soak the denture(s) in solution for 15 minutes twice daily. For maximum benefit, eating, drinking or rinsing should be avoided for at least 15-30 minutes after use. Contraindications: Use of the product by individuals hypersensitive to any of its ingredients should be avoided. Special Warnings and Precautions for use: For oral use only. Not to be swallowed. Keep away from the eyes and ears. If the solution comes into contact with the eyes, rinse well with water. Keep out of the reach and sight of children. If symptoms persist, stop using and consult your doctor or dentist. Chlorhexidine very rarely is known to induce hypersensitivity, including generalised allergic reactions and anaphylactic shock. It has been recommended that at least 30 minutes should be allowed to elapse between the use of toothpaste and oral chlorhexidine preparations. Use in specific populations: Pregnant or Lactating Women- No harmful effects in human pregnancy or during lactation have been reported. Adverse Reactions: Skin disorders: Allergic skin reactions such as dermatitis, pruritus, erythema, eczema, rash, urticaria, skin irritation, and blisters. Immune disorders: Hypersensitivity including anaphylactic shock. A superficial discoloration of the dorsum of the tongue may occur which disappears after discontinuation of treatment. Discoloration of the teeth and silicate or composite restorations may also occur. Transient disturbances of taste and a burning sensation of the tongue may occur on initial use of the mouthwash but usually diminishes with continued use. Discontinue the treatment in cases of oral desquamation or swelling of the parotid glands reported rarely. In all cases spontaneous resolution has occurred on discontinuation of treatment. Over dosage: Causes mucosal irritation and rarely systemic toxicity. In acute overdosage, zinc salts are corrosive; treatment consists of administration of milk or alkali carbonates and activated charcoal. The use of emetics or gastric lavage should be avoided. In case of accidentally swallowing more amount than to be used, professional assistance is advised.
Dated: 8th August, 2021
Further information is available on request.
GGI-CO-A1-AQS-300026984-WM-D22-0256