BixiBat’s Advantages in Laxative Therapy Explained, Chronic constipation, Elobixibat, General Practitioner, Consultant Physician, General Practice, Constipation Management, Gastroenterology, Gastroenterologist, Consultant Physician, SBM: Spontaneous Bowel Movement, CSBM: Complete Spontaneous Bowel Movement
This article is brought to you by Bixibat

This Article is brought to you by BixiBat
Elobixibat’s Unique Benefits in the Laxative Landscape:
A Comparative Overview
![]()
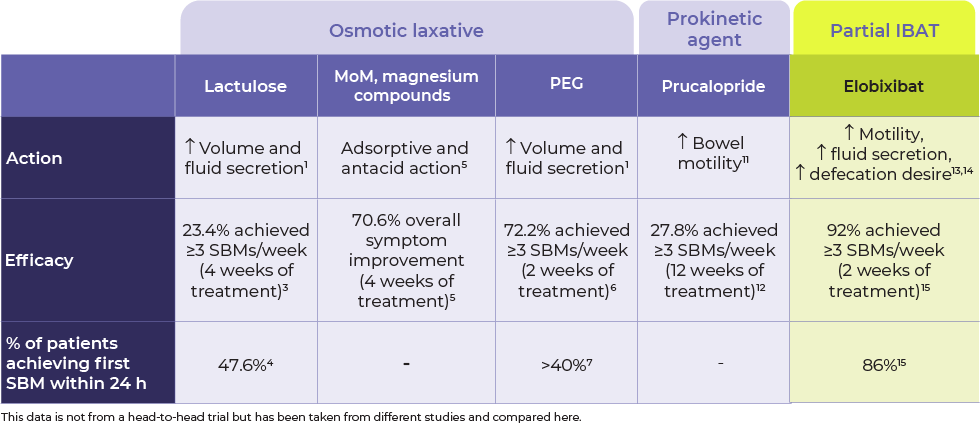


CI: Confidence interval; CSBM: Complete spontaneous bowel movement; MoM: Milk of magnesia; n/a: Not available; NNT: Number needed to treat; NSAID: Nonsteroidal anti-inflammatory drug; PEG: Polyethylene glycol; SBM: Spontaneous bowel movement.
References: 1. Balekuduru A, Sahu MK. Habit forming properties of laxatives for chronic constipation: A review [version 2; peer review: 1 not approved]. F1000Research. 2023;11:803. 2. Sanis.com. LACTULOSE (Lactulose solution 667 mg/mL). Available at: https://pdf.hres.ca/dpd_pm/00031381.PDF. Accessed on: 30 September 2024. 3. Kang SJ, Cho YS, Lee TH, et al. Medical management of constipation in elderly patients: Systematic review. J Neurogastroenterol Motil. 2021;27(4):495–512. 4. Kasugai K, Iwai H, Kuboyama N, et al. Efficacy and safety of a crystalline lactulose preparation (SK-1202) in Japanese patients with chronic constipation: A randomized, double-blind, placebo-controlled, dose-finding study. J Gastroenterol. 2019;54(6):530–540. 5. Mori H, Tack J, Suzuki H. Magnesium oxide in constipation. Nutrients. 2021;13(2):421. 6. DiPalma JA, DeRidder PH, Orlando RC, et al. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95(2):446–450. 7. Brenner DM, Corsetti M, Drossman D, et al. Perceptions, definitions, and therapeutic interventions for occasional constipation: A Rome working group consensus document. Clin Gastroenterol Hepatol. 2024;22(2):397–412. 8. Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut. 2000;46(4):522–526. 9. Health products regulatory authority. Sodium picosulfate ferring 10 mg tablets. Available at: https://www.hpra.ie/img/uploaded/swedocuments/LicenseSPC_PA1009-024-002_24052013132038.pdf. Accessed on: 30 September 2024. 10. Noergaard M, Traerup Andersen J, Jimenez-Solem E, et al. Long term treatment with stimulant laxatives - Clinical evidence for effectiveness and safety? Scand J Gastroenterol. 2019;54(1):27–34. 11. MOTEGRITY (prucalopride) tablets, for oral use. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210166s000lbl.pdf. Accessed on: 30 September 2024. 12. Camilleri M, Piessevaux H, Yiannakou Y, et al. Efficacy and safety of prucalopride in chronic constipation: An integrated analysis of six randomized, controlled clinical trials. Dig Dis Sci. 2016;61(8):2357–2372. 13. Acosta A, Camilleri M. Elobixibat and its potential role in chronic idiopathic constipation. Therap Adv Gastroenterol. 2014;7(4):167–175. 14. Manabe N, Umeyama M, Ishizaki S, et al. Elobixibat improves rectal sensation in patients with chronic constipation aged ≥60 years: A randomised placebo-controlled study. BMJ Open Gastroenterol. 2023;10(1):e001257. 15. Nakajima A, Taniguchi S, Kurosu S, et al. Efficacy, long-term safety, and impact on quality of life of elobixibat in more severe constipation: Post hoc analyses of two phase 3 trials in Japan. Neurogastroenterol Motil. 2019;31(5):e13571. 16. Mukherjee S, Patel P, John S. Lactulose. StatPearls. 2024. Available at: https://www.ncbi.nlm.nih.gov/books/NBK536930/. Accessed on: 30 September 2024. 17. Matsuyama M, Hirai K, Nonaka H, et al. Effects of elobixibat on constipation and lipid metabolism in patients with moderate to end-stage chronic kidney disease. Front Med (Lausanne). 2022;8:780127. 18. Schiller LR. Review article: The therapy of constipation. Aliment Pharmacol Ther. 2001;15(6):749–763. 19. Shono T, Hyakutake H. Efficacy and safety of elobixibat in hemodialysis patients with chronic constipation: A retrospective study. Ren Replace Ther. 2020;6:21. 20. Hishida Y, Nagai Y, Tsukiyama H, et al. Effects of elobixibat in patients with diabetes and concomitant chronic constipation: An 8-week, prospective, single-center, single-arm study. Adv Ther. 2022;39(9):4205–4217. 21. Włodarczyk J, Waśniewska A, Fichna J, et al. Current overview on clinical management of chronic constipation. J Clin Med. 2021;10(8):1738. 22. Fujisue K, Ito M, Matsuzawa Y, et al. Open-label, single-center, single-arm study evaluating the efficacy and safety of elobixibat for chronic constipation in patients with heart failure. Circ Rep. 2024;6(3):55–63. 23. Data on file. 24. Chedid V, Vijayvargiya P, Camilleri M. Elobixibat for the treatment of constipation. Expert Rev Gastroenterol Hepatol. 2018;12(10):951–960. 25. BIXIBAT Prescribing Information (Data on file). 26. Dulcolax Twelve Plus Pico Liquid. Electronic Medicines Compendium. Available at: https://www.medicines.org.uk/emc/ product/905/ smpc#about-medicine. Accessed on: 30 September 2024.
For the use of a Registered Medical Practitioner, Hospital or a Laboratory only. GGI-CO-A1-AQS-300041570-ELC-L24-0760




















